LED Technology Advances Endoscopy
Long life, stability and ease of integration make LEDs the preferred light source. Combining them with state-of-the-art microelectronics and optics enables new applications.
Endoscopy is a minimally invasive medical procedure used to view or image inside the human body for surgical and diagnostic applications (Figure 1). This method was first used in the 19th century and has expanded to a wider use today, with applications ranging from external organs such as the ear, nose and throat to complex endoscopy of the gastrointestinal tract, respiratory tract and spinal surgery.
Traditional endoscopes consisted of rigid tubes with transmitted light. Optics were used to collect the resulting image and direct it to the human eye. Such endoscopy was limited to short depths within the human body.
Endoscopic technology evolved to the use of flexible tubes with the advent of fiber optics, allowing deeper penetration into the body with minimal surgical intervention. Fiber optics also allowed more efficient light coupling, enabling precise illumination of the anatomy being observed. Eventually, electronic red, green and blue (RGB) cameras replaced the human eye as the detector, and illumination sources evolved to the use of broadband xenon and halogen bulbs.
More recently, LED technology is becoming the preferred light source for endoscopy because of its long life, stability, reliability and ease of integration into endoscopy units. Combined with the rapid evolution of microelectronics and optics, LED illumination has enabled new advances and applications in the field.
For several decades, xenon bulbs have been the gold standard in endoscopy systems. The bright, stable, broadband output and uniformity over a large spectrum make xenon ideal for many biomedical applications and has enabled numerous advances in endoscopy and deep-cavity surgical illumination. Xenon bulbs have a longer lifetime than the traditional filament lamp and superior performance to halogen.
Xenon lamps also display a favorable correlated color temperature, or CCT (Figure 2), and CRI (color rendering index), which is a measure of how well the light source can reproduce the color of objects being observed. Higher CRI correlates with more accurate color reproduction (Figure 3) preferred by medical professionals, enabling them to identify anatomy based on color contrast. Xenon provides the perfect illumination for viewing the true colors of tissue and vasculature in the surgical field, which assists with improved accuracy in diagnosis and treatment.
Xenon also has the benefit of many years of innovation, along with an extremely broad output range — typically from 80 W to well over 400 W. The main drawback to xenon has been the lifetime of the light source or lamp, which usually ranges from 500 to 1000 hours of unit operation.
White LED light engines have gained popularity in endoscopic systems because of advances in LED brightness and a relatively broadband wavelength of white light. LED technology allows some customization of the CCT during manufacturing, as well as a stable CCT over the lifetime of the LED. Its relatively long lifespan eliminates the requirement to change xenon sources during the life cycle of the endoscopy system.
More modern LED systems allow for active CCT adjustment in the field. Having the white light consist of separate RGB lasers or LEDs allows the user to adjust the level of each visible color to their comfortable white or CCT level. This variable feature reduces surgeons’ eyestrain and optimizes contrast of the tissue morphology under observation.
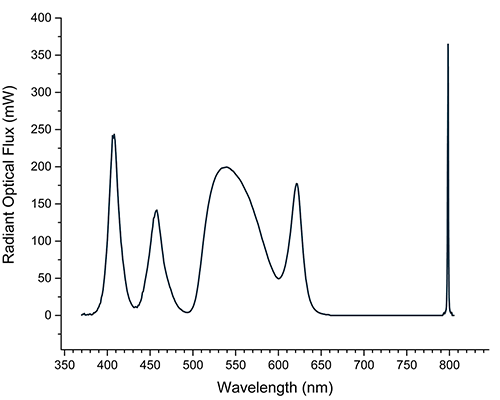
The LED systems also can couple effectively with current RGB cameras, as the RGB wavelength can be precisely tuned for optimum signal-to-noise ratio (Figure 4). Additionally, the ability to shape the spectrum allows achievement of different CCT and CRI values best suited to the camera or the eye. Levels of each color can be adjusted over the lifetime of the instrument to balance the colors as the intensity of each color degrades with age.
Autofluorescence was a method developed in the late 1990s to highlight areas of interest or contrast by exciting intrinsic fluorescence of cells and tissues. There is a difference in the emission spectrum between normal and cancerous tissues excited by blue light believed to be caused by altered chemical composition in physiology because of disease.
The early 2000s saw the advent of chromoendoscopy, a technique that uses traditional histological dyes to stain areas of interest during an endoscopic procedure. Typically, dyes such as methylene blue, toluidine blue and crystal violet are used. Each has an ability to stain a particular type of cell, allowing for easier identification of structures in mucosa and tissue.
Both chromoendoscopy and autofluorescent endoscopy assist in differentiating morphology when compared to conventional endoscopy techniques. While these are more sensitive than traditional wide-field endoscopy, they are also nonspecific, leading to increased false positive diagnoses.
Fluorescent biomarkers are gaining popularity in distinguishing morphology or recognizing diseased tissue in a surgical setting. One such marker known as ICG (indocyanine green) has been approved by the FDA for surgical use.
ICG stains plasma proteins. It targets lymph nodes and blood vessels, including those that invade tumors, guiding the surgeon to enable resection of diseased tissue that would otherwise be challenging under normal visible light. ICG fluorescence is excited by infrared light, which has a deeper penetration than visible light, allowing for visualization in deep tissue and layers of the retina.
Another compound known as ALA (aminolevulinic acid) has recently been approved for surgical use by the FDA. ALA is a compound naturally occurring in cells. Exogenous administration of ALA leads to accumulation of the ALA metabolite PpIX in tumor cells, specifically gliomas.
PpIX fluoresces when excited with 400- to 410-nm illumination, emitting red fluorescence. Increased PpIX fluorescence can then be used to guide the surgeon in differentiating tumor tissue (red) from normal tissue (blue) and enable precise and complete resection of the tumor.
Fluorescence technology requires specific wavelengths to excite the fluorescence biomarkers in question. Recent advances allow narrow wavelengths provided by LEDs or lasers to follow the same optical path as the white or viewing illumination (Figure 5). This allows both visible and otherwise invisible fluorescence information for the surgeon’s review.
Using visible light enables the surgeon to visualize anatomical areas of interest, followed by excitation of fluorescent biomarkers to aid in identification or resection of tissue. Having both visible and fluorescence capabilities available in one unit arms surgeons with multiple technologies to use for better diagnoses and improved patient outcomes.
Technology and contrast agents have evolved over the years, equipping surgeons and doctors with tools to assist them in complex diagnostic and medical procedures. Contrast agents can include white-light dyes, intrinsic autofluorescence or fluorescence markers. The challenge in development of these markers is in the decades spent in clinical trials to ensure minimal toxicity.
Fluorescence technologies are advancing as fast as LED and laser technologies. Studies are ongoing to identify molecules that will target specific tissue and provide ample fluorescence for quantitative rather than qualitative diagnosis and treatments. Using these technologies together is key to enabling precision in surgeries and decreased trauma to surrounding tissues, allowing for faster patient recovery times.